40 best lewis structures chemistry images in 2020
Table of Contents
Table of Contents
Are you struggling to understand how to draw Lewis dot structures for covalent compounds? Don’t worry, you’re not alone. Many students find this concept challenging, but with some practice and understanding, you can master it.
When it comes to drawing Lewis dot structures for covalent compounds, some common pain points include understanding the octet rule, knowing the number of valence electrons for each atom involved, and identifying which atom should be the central atom. These challenges can make the process confusing and overwhelming, but with some guidance, you can overcome them.
The first step towards drawing Lewis dot structures for covalent compounds is to determine the number of valence electrons each atom has. Valence electrons are electrons located in the outermost shell of an atom and are involved in bonding. Once you have determined the number of valence electrons for each atom, you can start arranging them around the central atom using the octet rule as a guide.
When following the octet rule, each atom should have eight electrons in its outer shell, except for hydrogen, which should have two. The central atom is typically the least electronegative atom, and its valence electrons are drawn as dots around it. For the other atoms, their valence electrons are drawn as dots or lines to represent bonds between them and the central atom.
In summary, to draw Lewis dot structures for covalent compounds, you need to determine the number of valence electrons for each atom, identify the central atom, and follow the octet rule when arranging electrons around each atom. You should also remember that the central atom is usually the least electronegative atom.
How to Draw Lewis Dot Structures for Covalent Compounds: Tips for Success
When I was first learning how to draw Lewis dot structures for covalent compounds, I found it helpful to practice with simple molecules like water (H2O) and methane (CH4). These molecules have fewer atoms and bonds, so they are less complex and easier to manage.
Another tip is to memorize the number of valence electrons for each atom involved in covalent bonding. For example, carbon has four valence electrons, nitrogen has five, and oxygen has six. Knowing these numbers can help you determine how many electrons you need to place around each atom.
It’s also essential to pay attention to the charge on the molecule, as this can affect the number of electrons present. If the molecule has a positive charge, it’s because it lost electrons, and if it has a negative charge, it gained electrons. You’ll need to add or subtract electrons accordingly when drawing the Lewis dot structure.
Common Pitfalls to Avoid When Drawing Lewis Dot Structures for Covalent Compounds
One common mistake students make when drawing Lewis dot structures for covalent compounds is not following the octet rule for each atom. Remember that every atom except for hydrogen should have eight electrons in its outer shell. Also, be careful not to forget to account for the electrons from double or triple bonds.
Another pitfall to avoid is incorrectly identifying the central atom. Remember that the central atom is usually the least electronegative atom, and that will help you determine where to place the other atoms and electrons.
The Benefits of Understanding How to Draw Lewis Dot Structures for Covalent Compounds
Understanding how to draw Lewis dot structures for covalent compounds is essential for anyone studying chemistry. It provides a visual representation of the molecule and allows you to determine its shape, polarity, and reactivity. These factors play a crucial role in how a molecule interacts with other molecules in a reaction, allowing you to predict its behavior.
Practice Makes Perfect
Like with anything else in chemistry, practice is essential. The more you practice drawing Lewis dot structures for covalent compounds, the easier it will become. Start with simple molecules and work your way up to more complex ones. Don’t be afraid to make mistakes, and remember that it takes time and effort to master this skill.
Question and Answer
Q: How do I determine which atom is the central atom?
A: The central atom is usually the least electronegative atom. If two atoms have the same electronegativity, the atom with the most available valence electrons is the central atom.
Q: What is the octet rule?
A: The octet rule is a chemical rule that states that atoms tend to combine in such a way that they have eight electrons in their outermost electron shell. Hydrogen is an exception and only needs two electrons in its outer shell.
Q: Why is understanding how to draw Lewis dot structures for covalent compounds important?
A: Understanding how to draw Lewis dot structures for covalent compounds is important because it provides a foundation for understanding organic chemistry and how molecules interact in chemical reactions. It also allows you to predict a molecule’s shape, polarity, and reactivity.
Q: How can I practice drawing Lewis dot structures for covalent compounds?
A: You can practice drawing Lewis dot structures for covalent compounds by starting with simple molecules and working your way up to more complex ones. Use an online tool like ChemDraw or MolView to practice drawing structures and check your work.
Conclusion of How to Draw Lewis Dot Structures for Covalent Compounds
Drawing Lewis dot structures for covalent compounds can be challenging, but it’s an essential skill to master if you’re studying chemistry. By following the octet rule, determining the number of valence electrons for each atom, and identifying the central atom, you can draw accurate Lewis dot structures. With practice and perseverance, you’ll be drawing structures for complex molecules in no time.
Gallery
Drawing Lewis Dot Structures For Covalent Compounds - YouTube
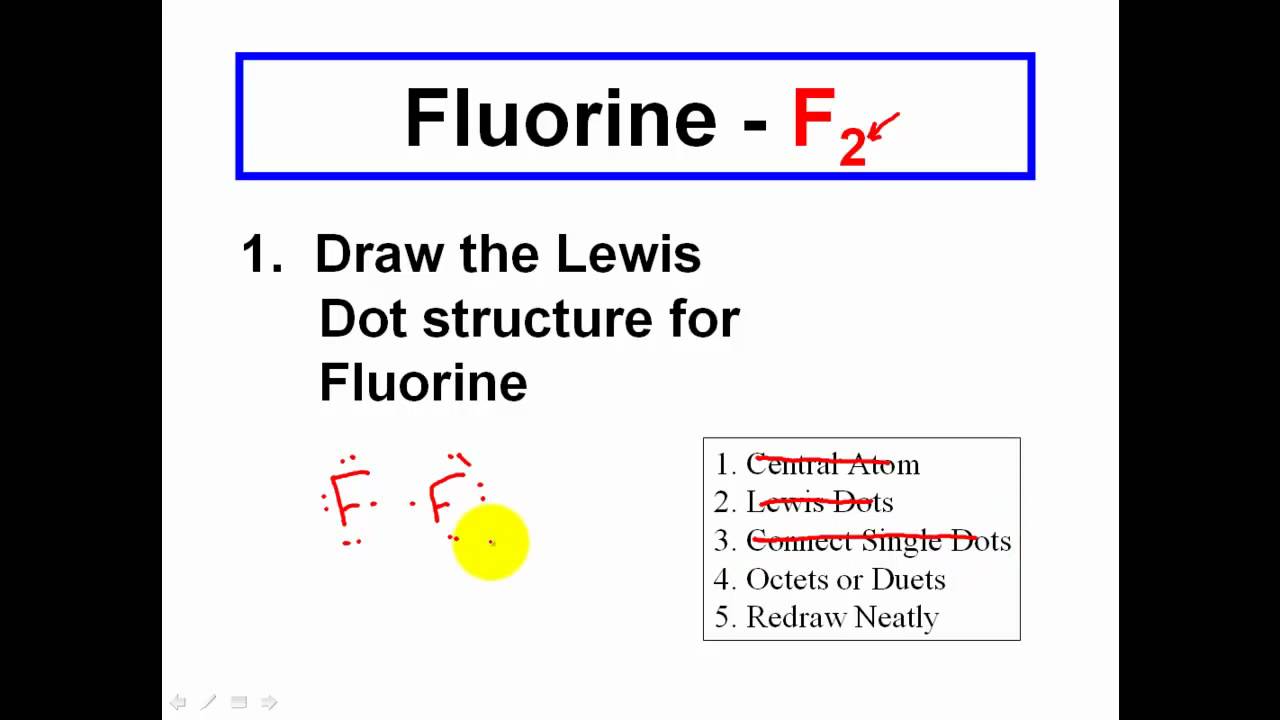
Photo Credit by: bing.com / lewis dot covalent compounds structures drawing
40 Best Lewis Structures Chemistry Images In 2020 | Chemistry, Teaching

Photo Credit by: bing.com /
Lewis Dot Diagram For Carbon Wiring Diagram | Free Nude Porn Photos
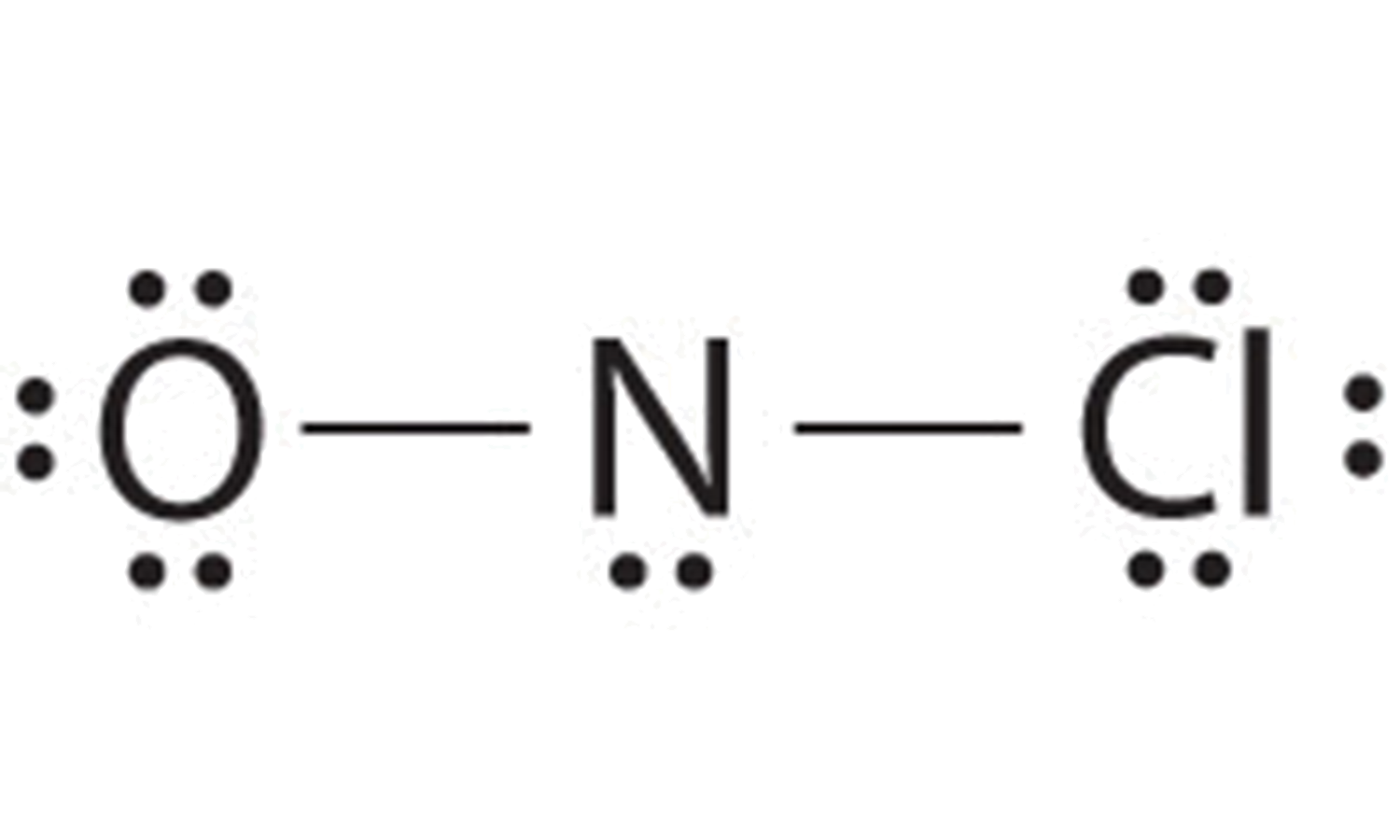
Photo Credit by: bing.com /
CHEMISTRY 101 - Draw Lewis Dot Structures For Covalent Compounds - YouTube

Photo Credit by: bing.com / lewis covalent dot compounds draw structures chemistry
A Single Carbon Atom Can Form A Maximum Of ______ Covalent Bond(s

Photo Credit by: bing.com / bond covalent lewis methane structure ch4 carbon single form maximum atom electrons labeled wps